aluminium electronic configuration|aluminum ground state electron configuration : Manila In order to write the Aluminium electron configuration we first need to know the number of electrons for the Al atom (there are 13 electrons). When we write the configuration . Draw attention to your garden with a beautiful statement piece with our high quality handcrafted glazed outdoor pot collection. A large variety of shapes and sizes are available. Skip to Header Skip to Main . Willory Ceramic Glazed Pot Large Fireworks Green $ 110 each. 4 interest free payments of $27.5 More info . Buy Online . Click & Collect .
PH0 · write an abbreviation configuration for aluminum
PH1 · silicon electronic configuration
PH2 · electronic structure of aluminium atom
PH3 · electronic configuration class 8
PH4 · electron configuration magnesium
PH5 · aluminum ground state electron configuration
PH6 · aluminium ion electron configuration
PH7 · aluminium electron arrangement
PH8 · Iba pa
Chimchar: Is a good pokemon with a really nice special movepool, really solid in many fights, also gets a nice support movepool for doubles. Litten: A very strong pokemon especially in doubles, it can still hold itself in singles with intimidate being great in the early game where most mons are physical.
aluminium electronic configuration*******Mar 23, 2023 Electronic configuration [Rn] 5f 14 6d 10 7s 2 7p 6: Crystal structure (predicted) FCC .In order to write the Aluminium electron configuration we first need to know the number of electrons for the Al atom (there are 13 electrons). When we write the configuration .
Electron Configuration of Aluminum. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Since aluminum's .
Aluminum Electron Configuration. A step-by-step description of how to write the electron configuration for Aluminum (Al). In order to write the Al electron .
The Electron configuration of aluminum is 1s22s22p63s23p1. Aluminum is one of the elements that make up the periodic table, which is distinguished by its symbol Al, and its atomic number 13. This element, .
Electron configuration of Aluminium is [Ne] 3s2 3p1. Possible oxidation states are -2; -1; +1; +2; +3. An aluminium atom has 13 electrons, arranged in an electron configuration of [Ne] 3s2 3p1, with three electrons .
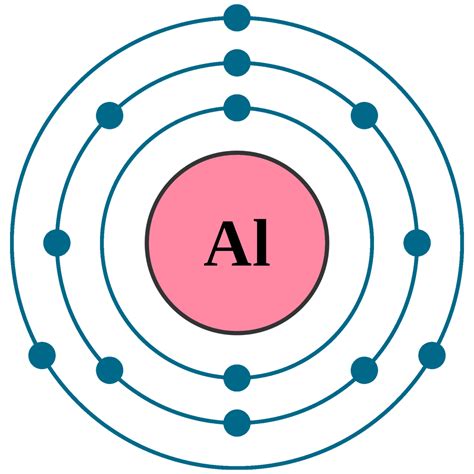
Learning Objectives. Describe how electrons are grouped within atoms. Write electron configurations for atoms. Connect the electron configuration of atoms to the . The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. .15.3.1: Electronic Configuration. Page ID. Pieter Kok. University of Sheffield. Table of contents. No headers. Often, but not always, the periodic table will include one or two . Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Aluminum . The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more .Q. Aluminum (Al) atom has ground-state electron configuration as : Q. What is electronic configuration .How to find the electronic configuration of metals as a ex.oxygen. Q. What are the valencies of the element given below. carbon. electronic configuration. 2,4. magnesium. electronic configuration. 2,8,2. oxygen. electronic .aluminium electronic configurationUn atome d’aluminium a 13 électrons, disposés dans une configuration électronique de [Ne] 3s2 3p1, avec trois électrons au-delà d’une configuration de gaz rare stable. L’aluminium peut relativement facilement céder ses trois électrons les plus externes dans de nombreuses réactions chimiques (voir ci-dessous). L . 1 Answer. Aluminium has atomic number 13 so the full electron configuration will be: 13Al 1s22s22p63s23p1. A shorthand way of writing this is to use the preceding noble gas configuration by putting its symbol in square brackets in front of the valence electrons. In this case that is neon which is:
Die Elektronenkonfiguration von Aluminium ist 1s22s22p63s23p1. Aluminium ist eines der Elemente des Periodensystems, das sich durch sein Symbol Al und seine Ordnungszahl 13 auszeichnet. Dieses Element mit 13 Elektronen und 13 Protonen ist ein nicht ferromagnetisches Metall, das zu den ersten drei häufigsten Elementen in der Erdkruste .
The aluminium atom contains 13 electrons. The electronic configuration of the aluminium is expressed in the form of the diagram as given below-. 1s orbital having minimum energy is filled first, with a maximum capacity of two electrons. After 1s orbital, the 2s orbital is filled with a maximum capacity of two electrons. La configuration électronique non abrégée de l'aluminium se compose d'un total de 13 électrons qui sont remplis comme suit -. Deux électrons sont présents dans l'orbite 1s. Deux électrons en orbite 2s. Six électrons en orbite 2p. Deux électrons en orbite 3s. Un électron en orbite 3p.
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). Summary. Aluminum is a chemical element. Its symbol is Al. it is almost impossible to find pure aluminum in nature because it is such a highly reactive element. Aluminum is not a magnetic material. Aluminum’s atomic number in the periodic table of elements is 13. The electron configuration of Aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1.
Electronic configuration. Aluminium has 13 electrons and 14 neutrons. The proton number is also 13. The electron configuration for Aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1 . Aluminium has 3 electrons in .aluminium electronic configuration aluminum ground state electron configurationAluminium (Aluminum in North American English) is a chemical element; it has symbol . Such an electron configuration is shared with the other well-characterized members of its group, boron, gallium, indium, and . The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more .
Let's find the ground state electron configuration of Aluminum! A single Aluminum atom has 13 protons and 13 electrons, but how do we know where Aluminum put. Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.

The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .
aluminum ground state electron configuration The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more .
Catryona Lei is on Alua! Catryona Lei invites you to join her on Alua and enjoy her exclusive content. She is a babygirl who loves to show off her curves and tattoos. Don't miss her sexy and playful photos and videos. Subscribe now and get .
aluminium electronic configuration|aluminum ground state electron configuration